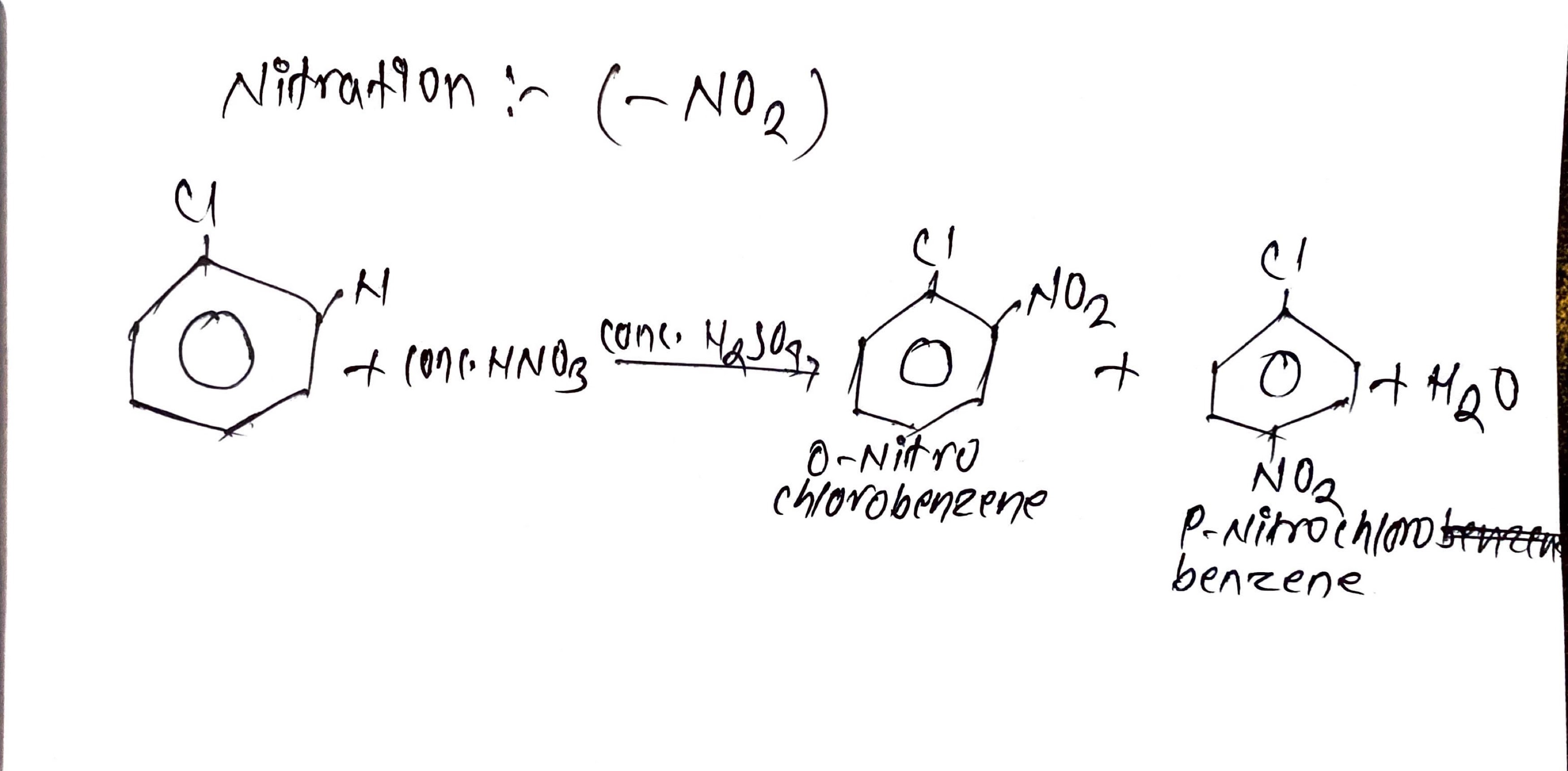| Unit 9: Haloarenes |
|---|
| 9.1 Describe briefly the nomenclature and isomerism of haloarenes. |
| 9.2 Show the preparation of chlorobenzene from benzene and benzene diazonium chloride. |
| 9.3 State physical properties of haloarenes. |
| 9.4 Describe the low reactivity of haloarenes as compared to haloalkanes in terms of nucleophilic substitution reaction. |
| Reaction (i) With NaOH, Zinc dust (ii) With Ammonia (iii) With copper cyanide |
| 9.5 Explain the chemical properties of haloarenes: reduction of chlorobenzene, electrophilic substitution reactions, action with Na (Fittig and Wurtz-Fittig), and action with chloral. |
| 9.6 Describe the uses of haloarenes. |
Haloarenes (Aryl Halides)
Haloarenes is a derivative of aromatic hydrocarbon because it is derived by replacing the hydrogen of aromatic hydrocarbon with a halogen atom. or
The group of organic compounds in which the halogen atom is directly attached to the benzene ring.
Use: Pesticide like DDT, Solvent, To prepare Aniline, Phenol, Chlorotoluene, etc.
Nomenclature
Isomerism
Compounds having the same molecular formula but different in structure are called Isomers and the Phenomenon is called Isomerism.
Read More: Haloalkanes Notes Class 12 Chemistry
Preparation of Chlorobenzene
1. From Benzene
When chlorine is passed into benzene in the presence of catalysts like Lewis acid (AlCl3, FeCl3) in dark conditions, it gives Chlorobenzene.
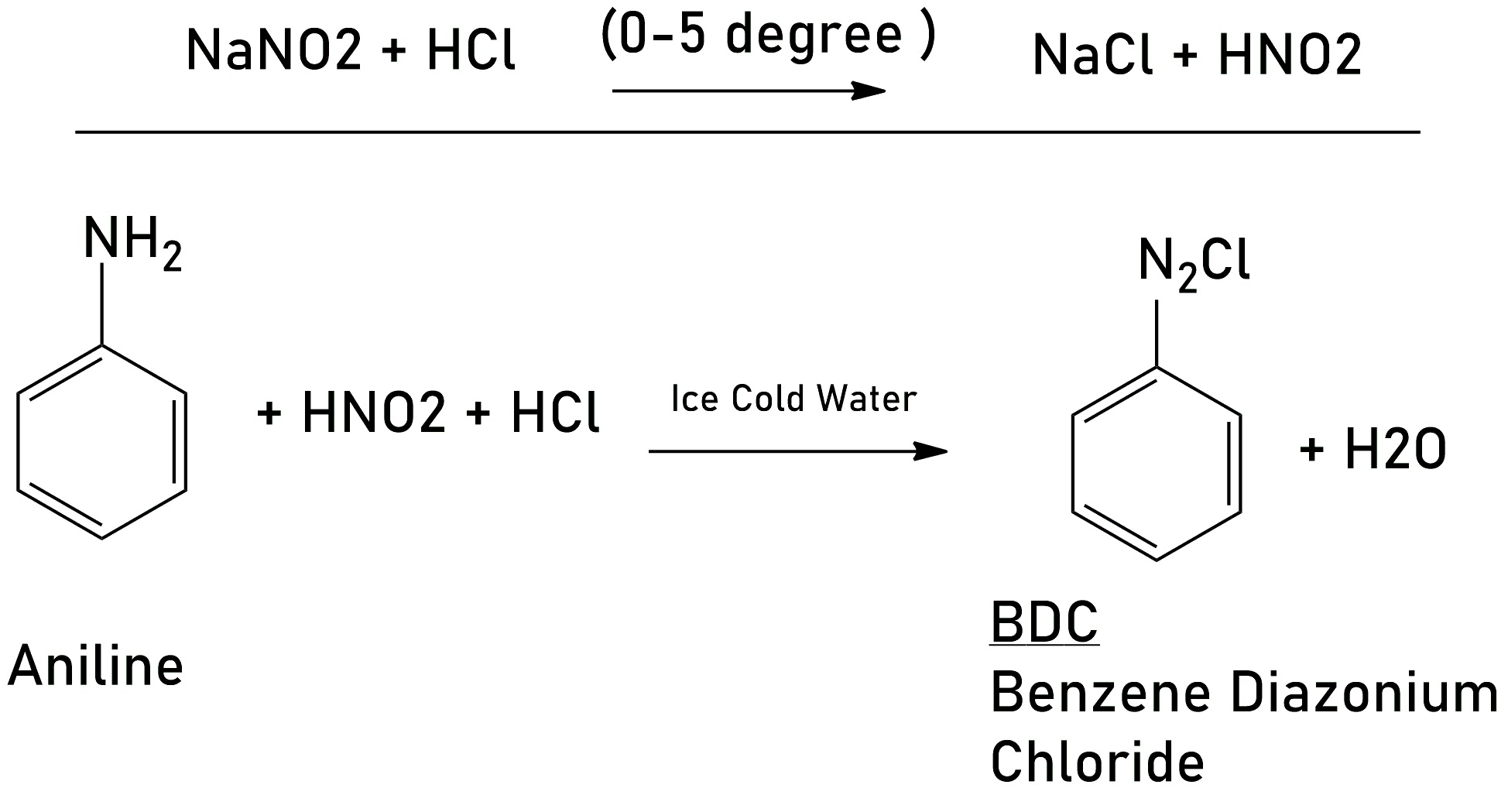
2. From Benzene Diazonium Chloride (BDC)
Preparation of BDC: Aniline reacts with nitrous acid ( NaNO2 -HCl) below the 5* degree it gives Benzene diazonium Chloride, This reaction is called Diazodigation reaction.
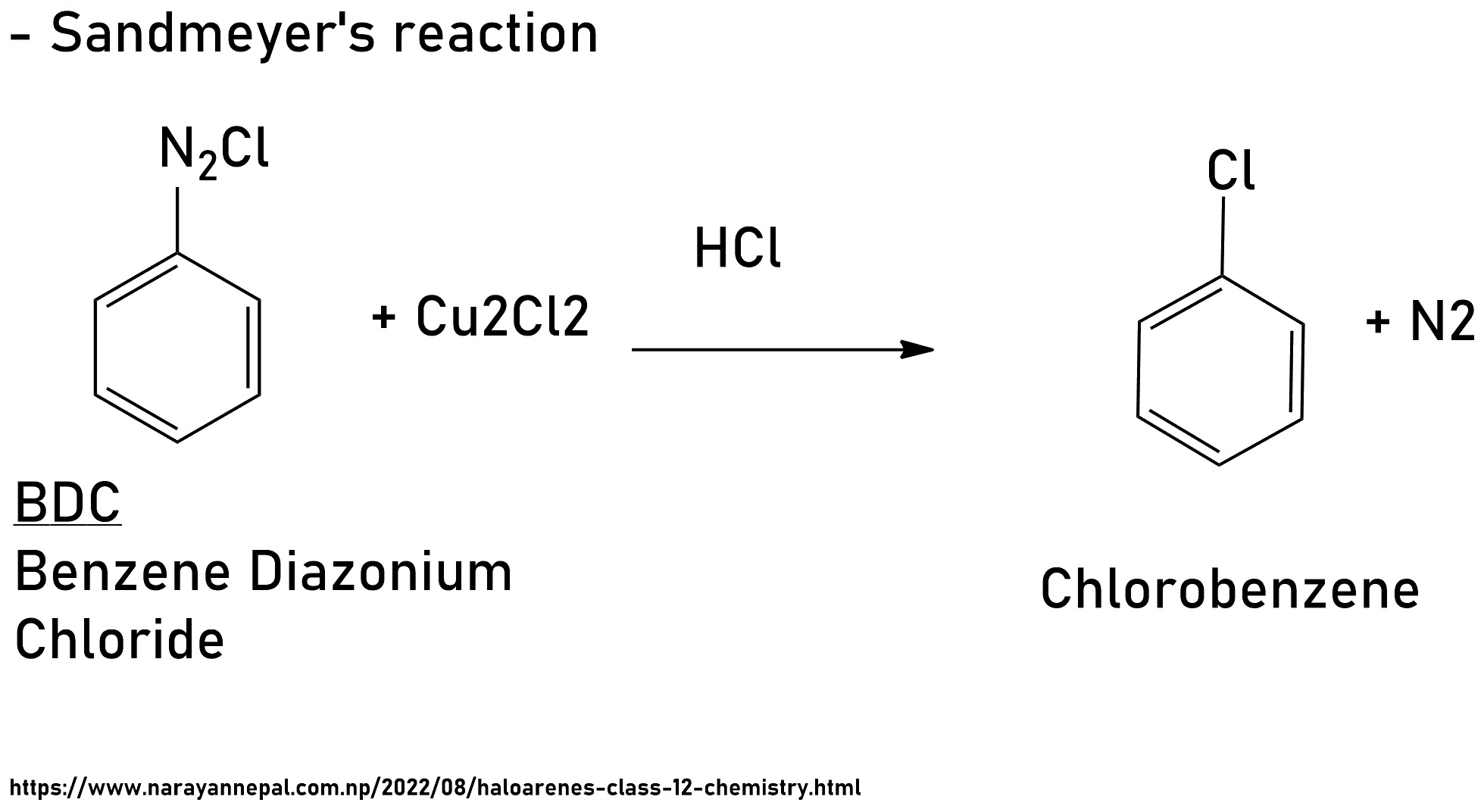
i) Sandmeyer's reaction:
BDC is warm with HCl, in the presence of Cu2Cl2 it gives Chlorobenzene. This Reaction is Sandmeyer's reaction.
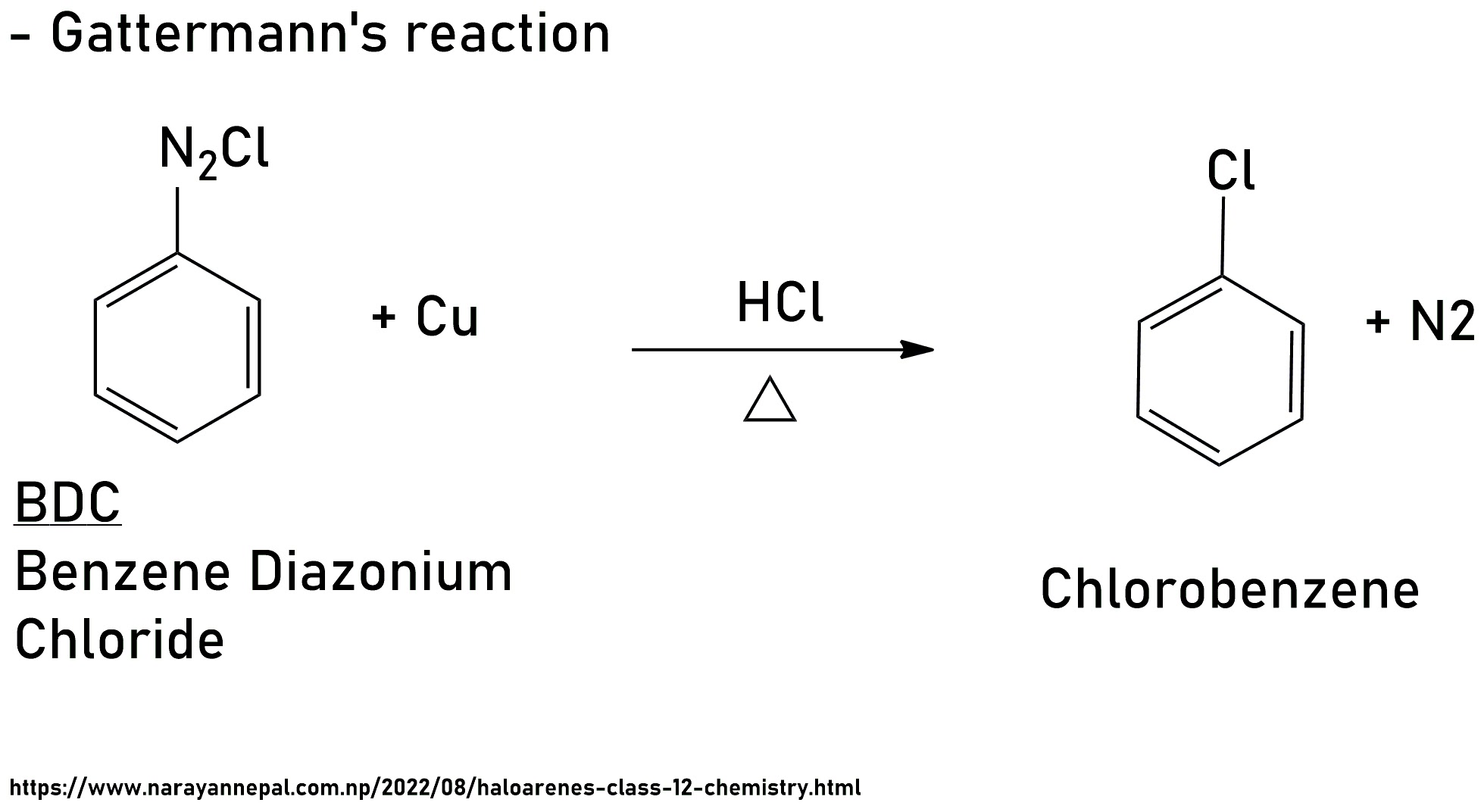
ii) Gattermann's reaction
BDC is heated with Cu in the presence of HCl it gives Chloro Benzene, this reaction in Called Gattermann's reaction.
Physical properties
1) State:
Haloarene is a colorless liquid/crystalline solid with aromatic order. Chlorobenzene is a colorless liquid with pleasant order.
2) Melting and boiling point:
Melting point/ boiling point increases with increasing order, the boiling/ melting of haloarene is higher than parent hydrocarbon and decreases in the following order.
C6H5I > C6H5Br > C6H5Cl
3) Solubility:
They are insoluble in water but highly soluble in organic solvents like ether, chloroform, alcohol, etc.
1. Nucleophilic substitution reaction
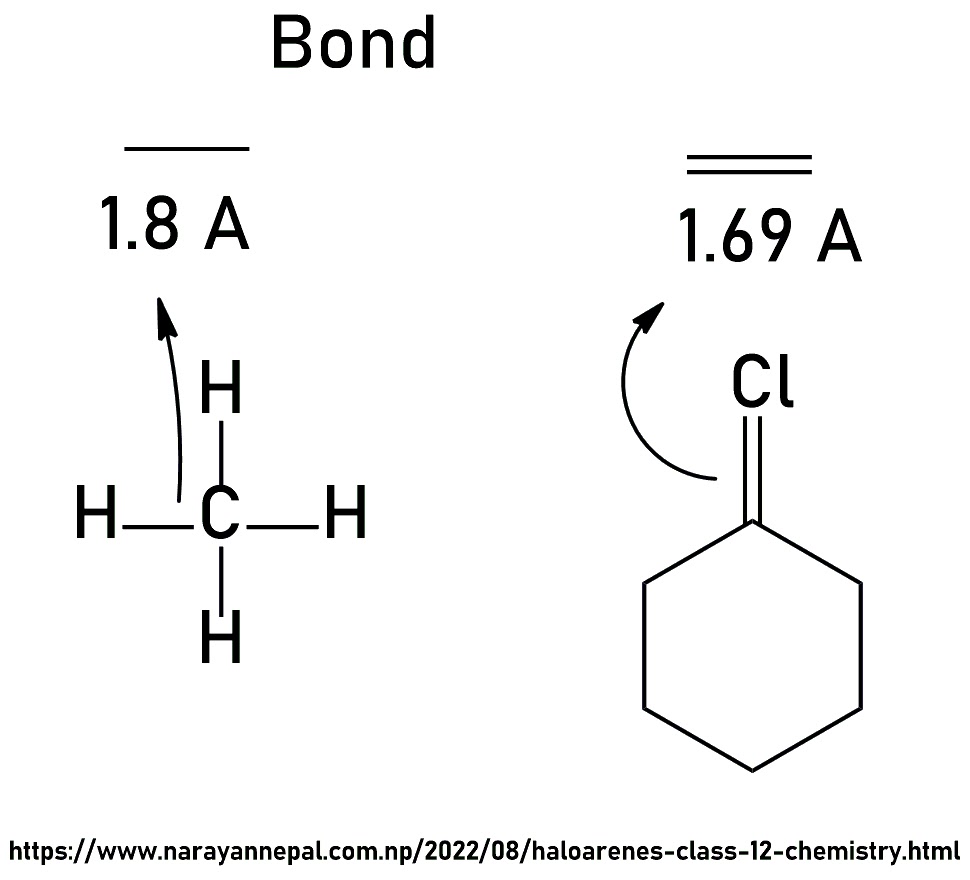
i. In terms of bond:
The reason for the low reactivity of haloarene over haloalkane towards the nucleophilic substitution is Resonance.
The above resonating structures 2nd, 3rd, and 4th show double bonds in C-Cl. A double bond (1.69A") is shorter than a single bond (1.8A") therefore, Haloarene is less reactive toward nucleophilic substitution reaction.
ii. In terms of hybridization
Carbon in Haloarene is SP2 hybridized and carbon in Haloalkane is SP3 hybridized. The carbon which has more S character is more electronegative and it can hold strongly halogen atoms.
In comparison to carbon which has less S character, therefore, Haloalkane is more reactive towards nucleophilic substitution reaction.
Reaction of Haloarenes
i. Reaction with NaOH:
When Chlorobenzene is heated with NaOH at 350'C under 300 atm pressure it gives sodium phenoxide which is acidified with HCl which gives Phenol.

Phenol can further heated in the presence of Zn dust with sodium chloride to give benzene.
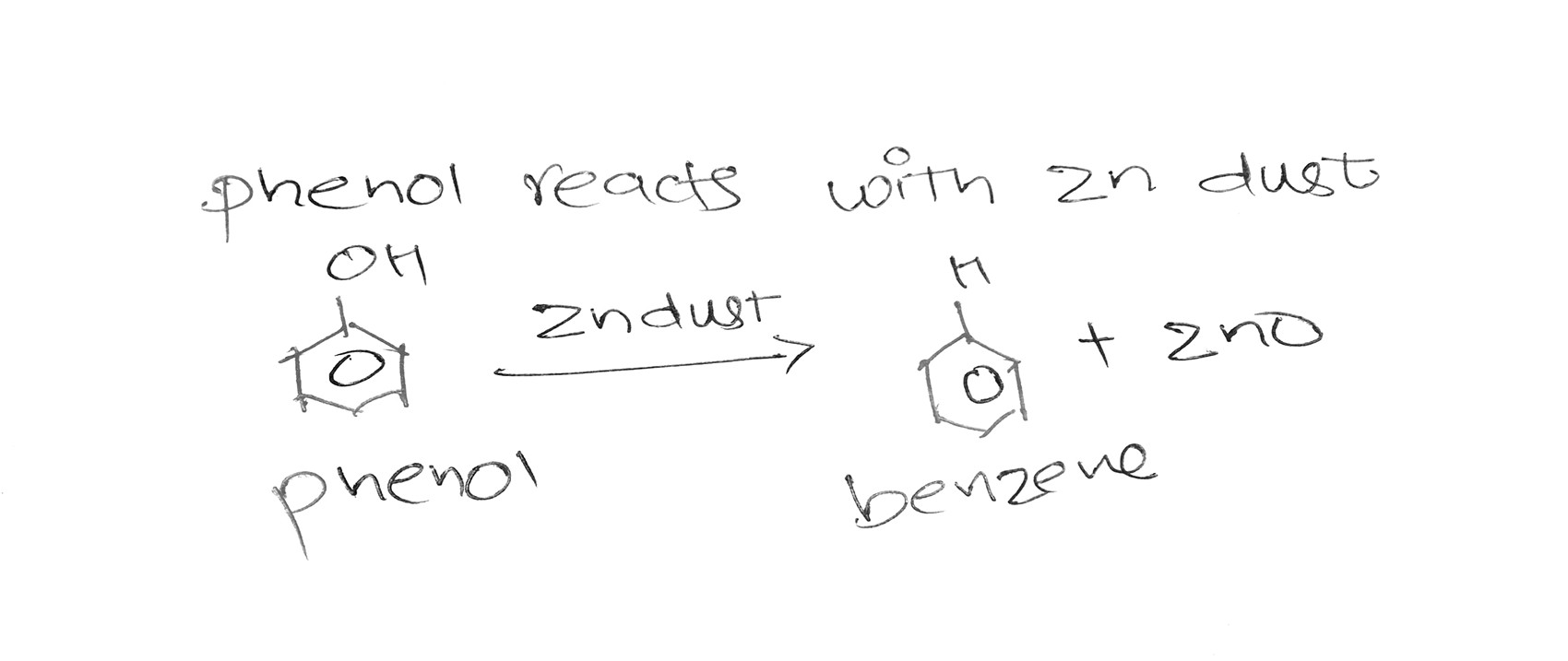
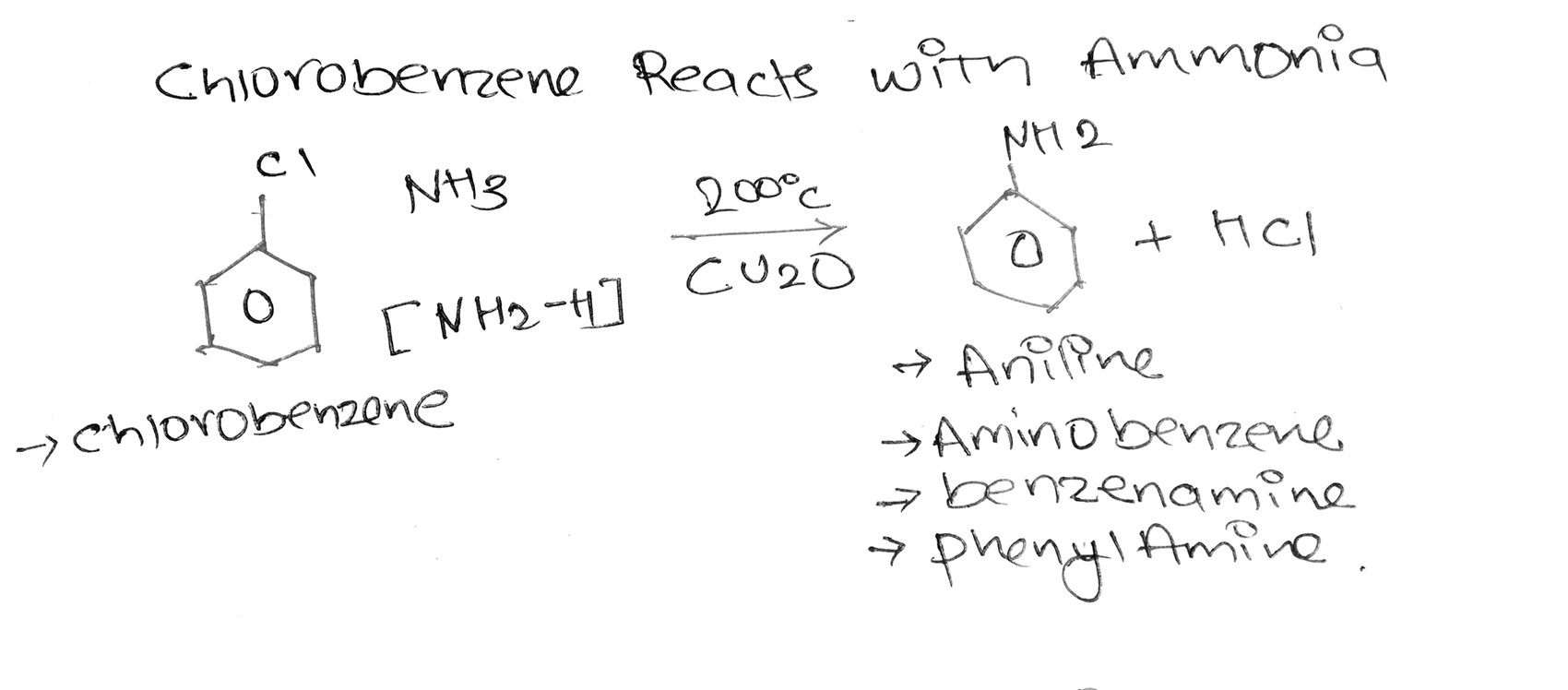
ii. Reaction with Ammonia
Chlorobenzene is heated with Ammonia at 200'C in the presence of Cuprous oxide (Cu2O), which gives Aniline.
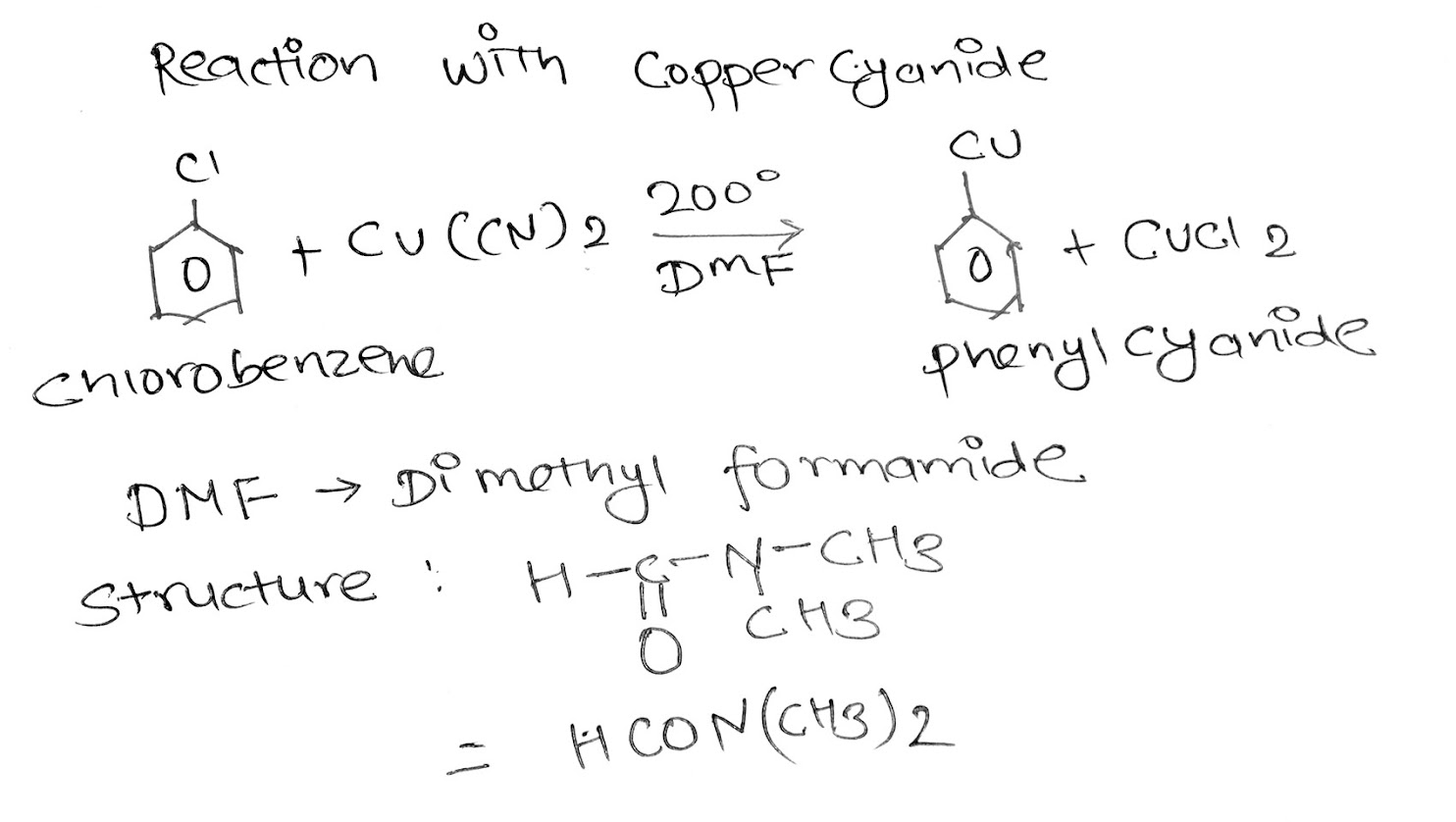
iii. Reaction with copper cyanide
When Chlorobenzene is heated with copper cyanide at 200'C in the presence of DMF it gives Phenyl cyanide.
2. Reduction reaction
When Chlorobenzene is heated with Ni-Al alloy in the presence of NaOH it reduces to give benzene.
3. Electrophilic substitution reaction
(reaction due to benzene ring)


In the above Resonating structures 2nd, 3rd, and 4th show high electron density at the ortho and para position, when it undergoes electrophilic substitution reaction, electrophile attacks at the ortho and para position to give ortho & para-substituted.
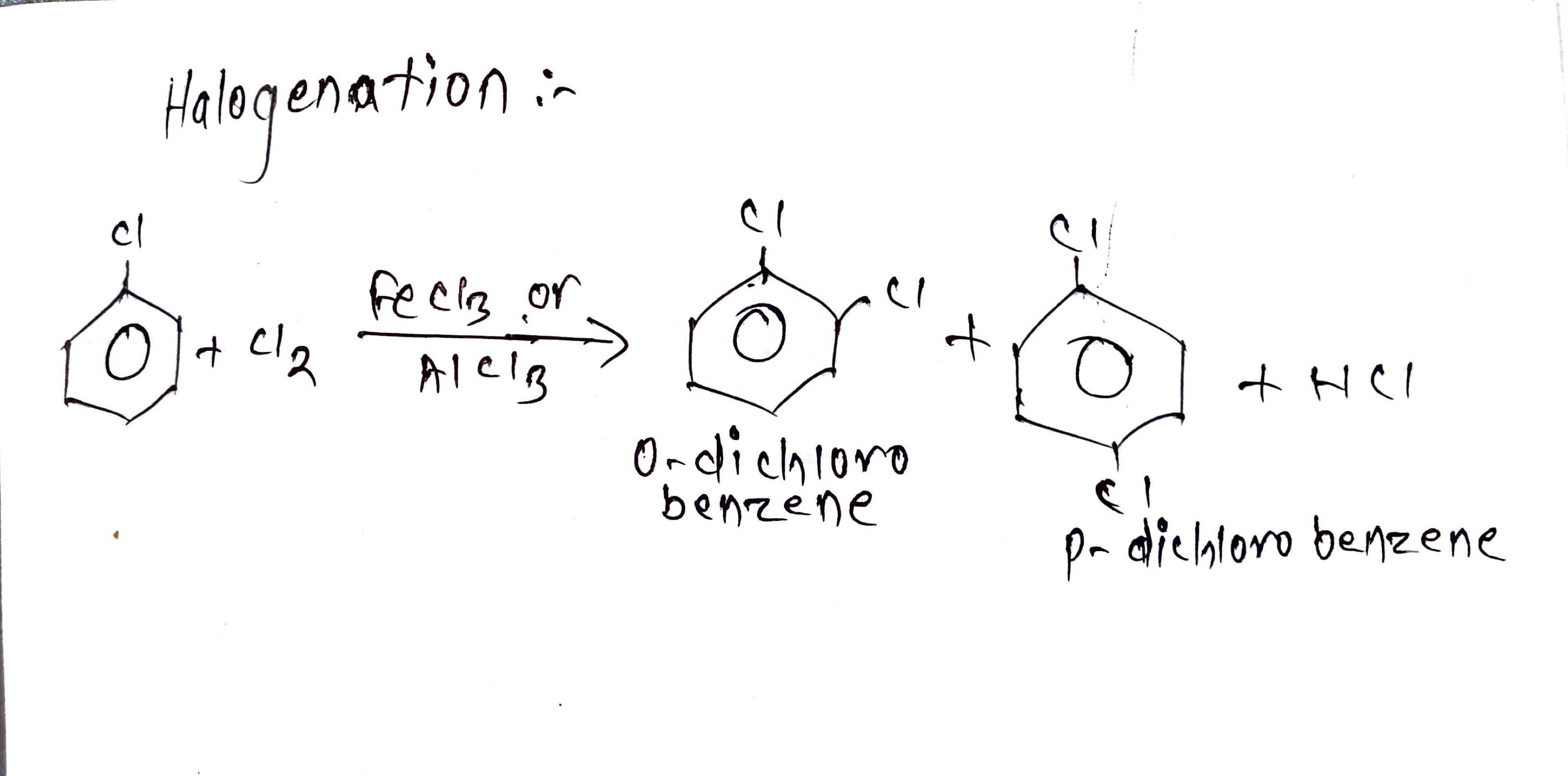
i. Halogenation:
Introducing halogen in the benzene ring by substituting hydrogen for halogen is called halogenation.
Chlorine is passed into chlorobenzene in the presence of FeCl3 or AlCl3 it gives ortho- di chlorobenzene and para - di chlorobenzene.
ii. Nitration (-NO2):
Chlorobenzene is heated with conc.HNO3 and conc.H2SO4 to give ortho Nitro chlorobenzene and para Nitro chlorobenzene.
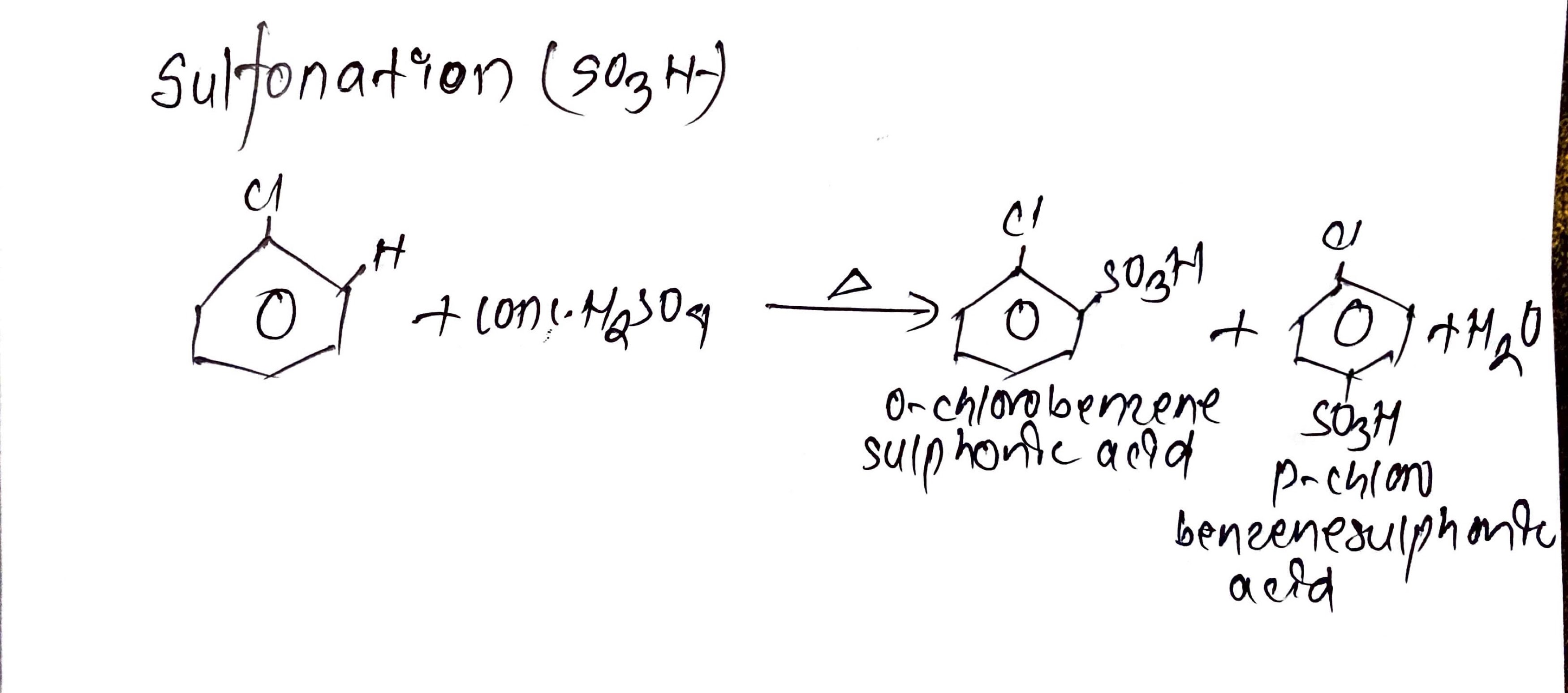
iii. Sulfonation(So3H):
Chlorobenzene is heated with conc.H2SO4 which gives Ortho- chlorobenzene sulphonic acid and para- chlorobenzene sulphonic acid.
iv. Friedel Craft's Alkylation (R- ):
Chlorobenzene is heated with Haloalkane (Alkyl halide) in the presence of anhydrous AlCl3, it gives ortho chlorotoluene and para chlorotoluene.
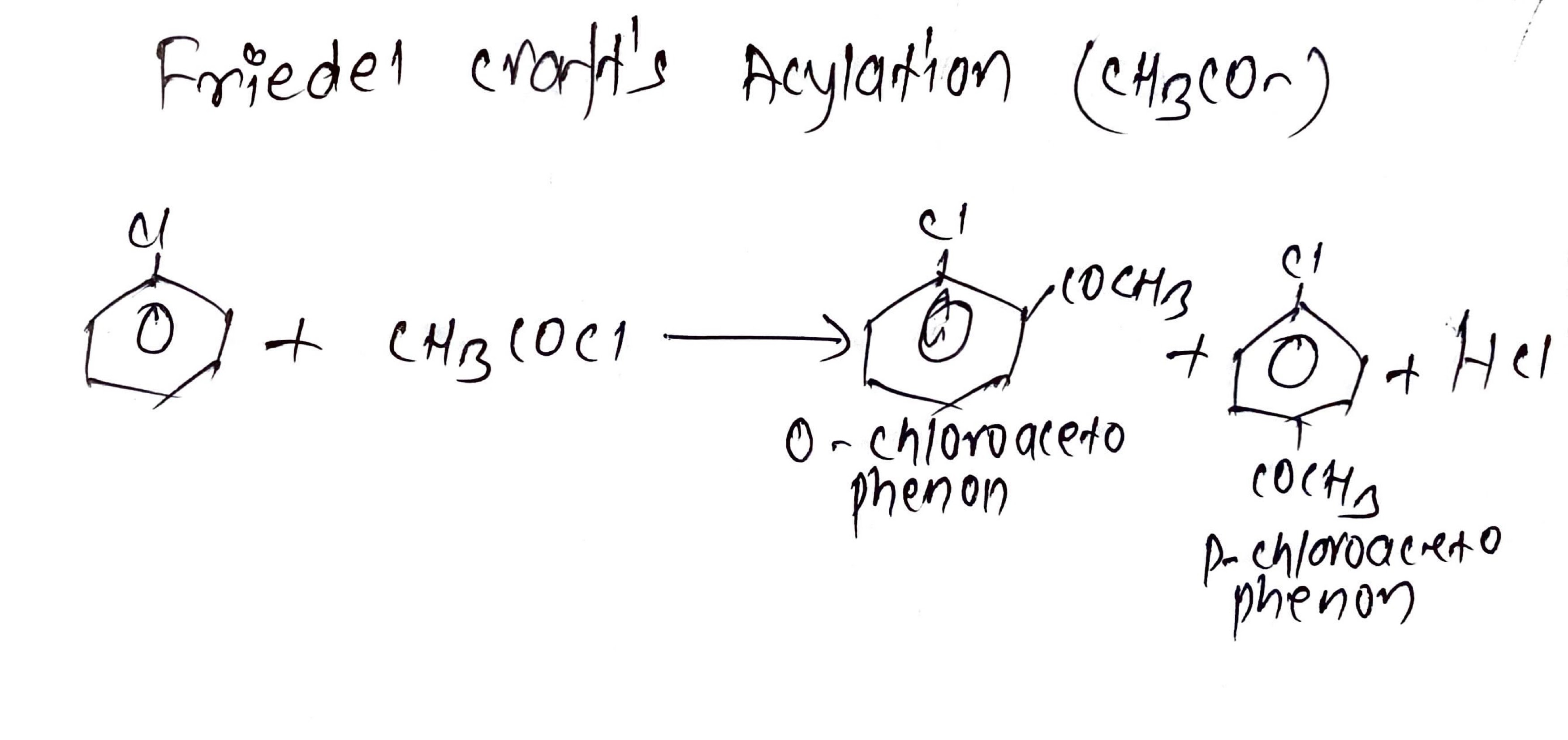
v. Friedel craft's Acylation (CH3CO- ):
Chlorobenzene is heated with acetyl chloride or acetic anhydride in the presence of anhydrous Aluminum Chloride (AlCl3) it gives Ortho- Chloroacetophenone and Para- Chloroacetophenone.
vi. Action with Choral
( Preparation of DDT)
4. Reaction with Metal ( sodium)
i. Fittig Reaction:
Chlorobenzene is heated with sodium in the presence of dry ether which gives Biphenyl.
ii. Wurtz–Fittig reaction:
When Chlorobenzene is heated with Alkyl halide with ethereal sodium it gives Alkylbenzene.
Uses of Haloarene
- Use to prepare Phenol, Aniline, Chlorotoluene, biphenyl, etc.
- Manufacture Insecticides and Pesticides like DDT.
- Organic synthesis is used to make other chemical compounds.
- Used as a fiber-swelling agent in textile processing